
However, there is still controversy as to which fluid achieves the best results clinically and experimentally. Pressure-controlled ventilation reduces P (a-ET)CO2 gradient, Paw and improves Cdyn but does not affect P (A-a) O2 or haemodynamics in comparison to volume-controlled ventilation in robotic surgeries in the Trendelenburg position.ī> Background: Resuscitation is the initial step for hemorrhagic shock. Haemodynamics and P (A-a)O2 were comparable between the groups. The Paw was lower at T1, T2, and T3 and Cdyn higher at T3 and Te in Group PCV at comparable minute ventilation. The P (a-ET)CO2 at T1, T2, T3 and at Te was lower in Group PCV versus Group VCV. The P (A-a) O2, Paw, Cdyn, heart rate and blood pressure were also measured at the same time. The P (a-ET)CO2 was measured at baseline T0, 10 min after Trendelenburg position T1, 2 h of surgery T2, 4 h T3 and at Te, 10 min after deflation. The effects on alveolar to arterial oxygen gradient P (A-a)O2, peak airway pressure (Paw), dynamic compliance (Cdyn) and haemodynamics were also assessed.įifty-one patients, 18-75 y, American Society of Anesthesiologists I-III undergoing robotic surgery in Trendelenburg position were randomised to volume-controlled ventilation (Group VCV) or pressure-controlled ventilation (Group PCV).

The primary objective was to assess the impact of two ventilatory strategies, volume versus pressure-controlled ventilation on the arterial to end-tidal carbon dioxide gradient P (a-ET)CO2 in patients undergoing robotic surgery in the Trendelenburg position.
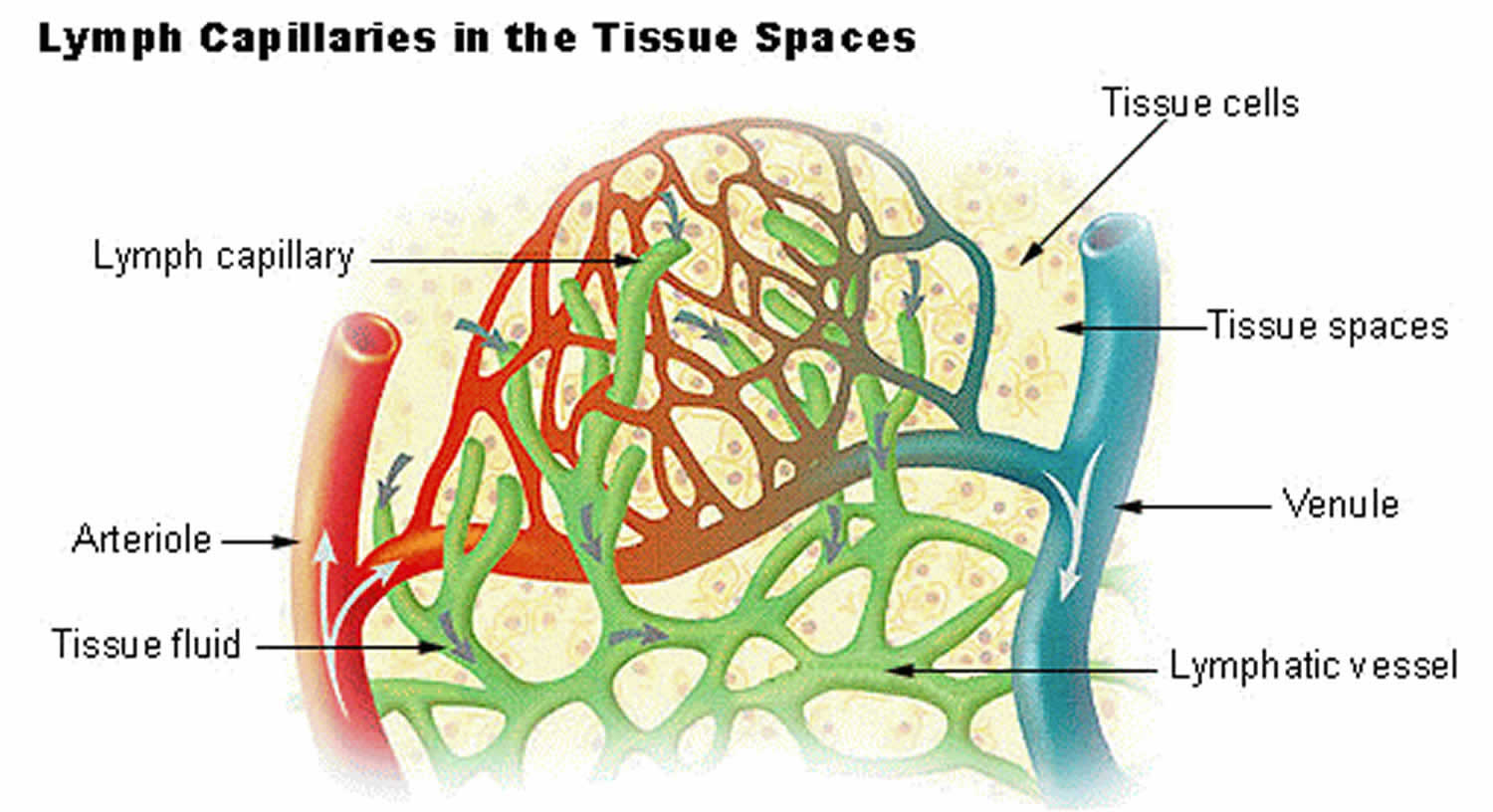
Steep head-down positions in pelvic surgery can increase the ventilation-perfusion mismatch and increase ventilatory requirements to offset carbon dioxide (CO2) increases consequent to pneumoperitoneum. Robotic surgery is increasingly prevalent as an advancement in care. These studies indicate that the mGAC is dynamically remodeled during monocyte activation and that mGAC remodeling itself may contribute to the heightened inflammation associated with AHF. Specifically, using RNA-seq, we found that enzymatic degradation of the mGAC increases transcription of inflammatory (IL6, CC元) and vascular (tissue factor/F3) mediators. Additionally, we found that ex vivo enzymatic removal of HS and disruption of the mGAC triggers human monocyte activation and amplifies monocyte inflammatory responses. Mechanistically, Toll-like receptor (TLR) activation of human monocytes results in GAC remodeling and a decrease in the mGAC (71% compared with no treatment p = 0.0007). In a cohort of hospitalized AHF patients (n = 17), we found that (1) the GAC degradation product heparan sulfate (HS) was elevated compared with age-matched controls (43 ng/mL p = 0.01) and that (2) HS and soluble CD14 (a marker of monocyte activation) levels were closely related (Pearson’s r = 0.65 p = 0.002). Flow cytometry was used to assess the mGAC and RNA-seq was employed to understand the role of the mGAC in regulating inflammatory activation programs. Inflammatory markers and GAC degradation products were profiled using ELISAs. In this study, we sought to determine if GAC degradation products are elevated in AHF patients, how these degradation products relate to circulating inflammatory mediators, and whether the monocyte GAC (mGAC) itself modulates monocyte activation. Much GAC research has focused on its role in vascular responses, with comparatively little known about how the GAC regulates immune cell function. The glycocalyx (GAC) is a sugar-based shell that envelops all mammalian cells. Acute heart failure (AHF) syndromes manifest increased inflammation and vascular dysfunction however, mechanisms that integrate the two in AHF remain largely unknown.


 0 kommentar(er)
0 kommentar(er)
